Fertiliser management is an innovative and science-based approach that targets environmental protection, increased agricultural production, increased profitability of agricultural production and sustainability. The concept involves applying the right source of fertiliser, at the right rates, at the right time and in the right place.
– this is the definition developed by the Fertilizer Institute. It is the optimisation of the use of nutrients that makes sustainable crop production possible and even necessary.
Managing soil nutrients requires knowledge of their content (soil abundance), dynamics of change and availability. It also requires knowledge of the function of the basic factors determining plant nutrient efficiency (water, pH, organic matter) and their interrelationship.
The nutrient richness of a soil depends on its mineral composition, grain fraction structure, quantity and quality of organic matter, soil pH, sorption properties including the ionic composition of the sorption complex. Soil resourcefulness resulting from natural soil-forming processes is natural resourcefulness. The abundance created by man through cultivation and fertilisation is referred to as agrotechnical abundance. All soil constituents have a specific availability to plants, which depends on many factors and, in addition to the availability of water, pH or temperature, may also depend on the species. Hence, a distinction can be made between overall abundance, i.e. the total amount of nutrients present in the soil, and abundance in substances available for specific species or even for specific plant stages.
The availability of nutrients to plants varies over time and depends on many factors, such as soil moisture, pH and temperature, the transformation of soil mineral and organic matter, soil microbial activity, the distribution of nutrients in the soil and others.
Water is a fundamental element in plant nutrition, as only in its presence is the transformation and uptake of nutrients possible. It is an environment for the exchange and transport of ions and is a prerequisite for the suitability of the soil for all forms of life. Its deficiency leads to a reduction in both soil fertility, fertility and productivity. Excess water shifts soil biology towards anaerobic transformation, acidification, and leads to the leaching of many nutrients.
Read more about the water function in Area 10: Water Management.
Soil reaction is one of the most neglected elements of agrotechnology in Poland. Currently, this applies to about 60% of our country’s land, which has a very acid and acid reaction, and only 18% of soils do not require liming.
The soil reaction (pH) determines the availability of macro- and micronutrients in the soil. Below pH 5, the availability of the most important macronutrients decreases considerably, and in the case of phosphorus, even below pH 6. At the same time, the availability of aluminium (Al+3) increases, and at pH values below 4.8, it has a phytotoxic effect on the development and proper functioning of the root system.
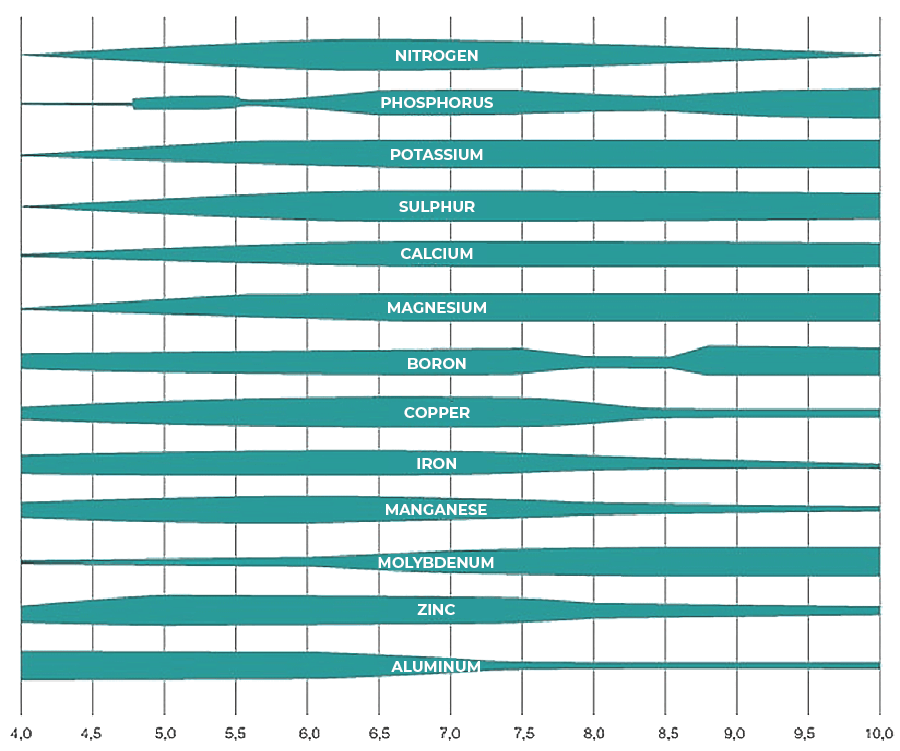
Due to reduced nutrient availability and the presence of harmful Al+3 ions, crops develop less well, resulting in a significant yield loss.
Yield loss rate in relation to soil pH range.
| Assessment of soil acidity | Range of pH | Range of yield loss, %. |
| Very acidic | <4.5 | 25 |
| Acidic | 4.6-5.5 | 15 |
| Slightly acidic | 5.6-65 | 5 |
| Indifferent | 6.6-7.2 | 1 |
| Alkaline | >7.2 | 2 |
Taking into account the surface area and acidification status of soils in Poland, we are annually failing to use approximately 114.6 thousand tonnes of nitrogen and 24.2 thousand tonnes of phosphorus potentially available to our crops. A significant proportion of nitrogen, due to leaching, enters surface and ground water, damaging the environment and the quality of drinking water. Phosphorus remains in the soils, becoming volatile at low pH to the form of crystalline minerals.
A European Parliament resolution (EP Resolution, 2007) mentions the decline in organic matter among the main threats to the proper functioning of soils, as its loss can cause many adverse phenomena related to the disruption of chemical, physical and biological processes that directly affect soil quality and its production capacity.
Soil organic matter, native and introduced in fertilisers, as well as the products of their biological and biochemical transformations, determine the composition of the entire soil complex, constituting its fertility and fertility. A high content of organic matter stabilises the soil structure and reduces its susceptibility to degradation by water and wind erosion. The humus compounds are capable of retaining up to five times the weight of water they themselves hold in a form that is accessible to plants. This is particularly important in sandy soils. Soil organic matter has a specific effect on the circulation of elements, increases the abundance, including up to 30 times its sorption capacity, has a positive effect on soil water management and its phytosanitary status. When using mineral fertilisers, the importance of organic matter lies less in the supply of nutrients and more in its buffering effect on nutrient concentrations, as well as in the processing of applied plant protection product substances.
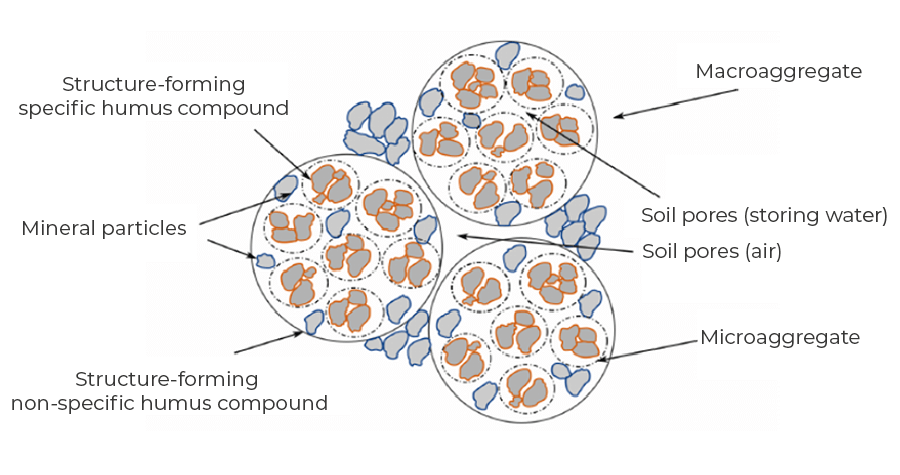
The dynamics of change in the abundance and availability of individual nutrients are highly variable and specific to each nutrient.
Nitrogen is present in the soil mainly (94%) in the form of organic compounds, the remaining 6% being nitrate and ammonium ions. However, their actual level is highly variable over time, depending on the season, rainfall, plant development and the decomposition time of their residues. A natural source of atmospheric fixed nitrogen is the non-symbiotic microorganisms Clostridium and Azotobacter, free-living microorganisms that can assimilate 10-15 kg N/ha per year, as well as the papillary bacteria Rhizobium, which, fixing atmospheric nitrogen in symbiosis with faba bean plants, usually provide 80-250 and sometimes up to 500 kg N/ha per year to the soil.
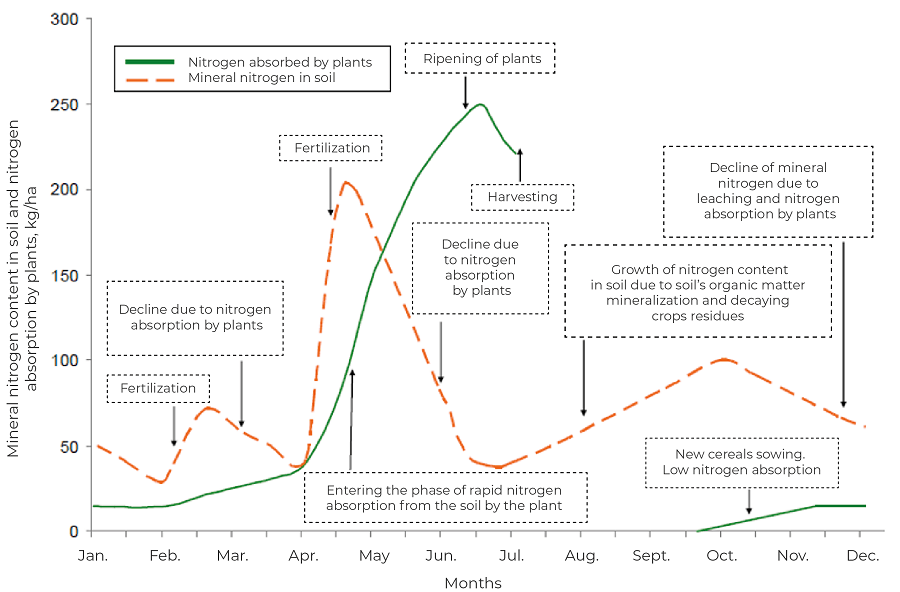
Nitrogen from mineral fertilisers and urea requires time and the right moisture and thermal conditions to convert into forms that are available to plant roots. It is therefore important to use soil fertilisers very thoughtfully and deliberately, and sometimes foliar fertilisation is necessary.
Phosphorus is an element that is not very mobile in the soil, either in mineral or organic compounds. It changes its chemical forms, depending on the pH and its ratio to carbon, to be more or less available to plants. Some of the phosphorus can leach into the profile, particularly in soils containing high amounts of organic matter or in acidic and very light soils.
Potassium binds to the mineral part of the sorption complex, but is easily leached, especially from light and organic soils. A deficiency of potassium drastically reduces nitrogen uptake, which lowers yields in a way that depends on the crop species. An adequate supply of potassium to plants promotes nitrogen uptake from the soil and improves the winter hardiness of plants. High doses of potassium displace calcium from the sorption complex and contribute to soil acidification. At low pH, potassium is poorly taken up by plants and is easily leached from the soil. High doses of potassium can also cause salinisation of the soil solution, especially during plant emergence, and excessive uptake of this element by plants.
Magnesium – high fertilisation with potassium and nitrogen, particularly in ammoniacal form, may result in increased magnesium ion leaching from the soil due to antagonistic effects, higher than its standard loss of 20 to 40 kg MgO/ha per year. Magnesium is particularly important for faba bean plants benefiting from symbiosis with Rhizobium bacteria, as under conditions of magnesium deficiency, the bacteria develop poorly and atmospheric nitrogen fixation is significantly reduced.
Calcium – annual losses of leached calcium are typically 200 to 1,500 kg CaO/ha, especially on light soils and in the absence of plant cover. Its loss leads to soil acidification, loss of soil structure and the development of micro-organisms unfavourable to crop plants.
Sulphur – proper plant nutrition with this nutrient promotes nitrogen uptake and thus increases nitrogen utilisation from soil/fertilisers, consequently reducing the risk of nitrate leaching into ground and surface water.
Information on nutrient management can be found in legislation: in the Act on Fertilisers and Fertilisation, the Act on Waste and in regulations of the Ministers of Agriculture, Economy and Environment. Recommendations and good practices can be found in publications developed with the participation of IUNG in Puławy, CDR in Brwinów or large fertiliser manufacturers, among others.
Practical consequences of unsustainable soil richness:
- excess of Mg results in removal from the soil and consequent deficiency of Ca, K,
- excess of K results in removal from the soil and consequent deficiency of Mg, Ca,
- excess of Ca results in removal from the soil and consequent deficiency of Fe, B, Mn, Mg, K,
- excess of PO4 results in the removal of Cu, Zn, Mn from the soil and consequent deficiency,
- excess of Fe results in inactivation in the soil and consequently a deficiency of P